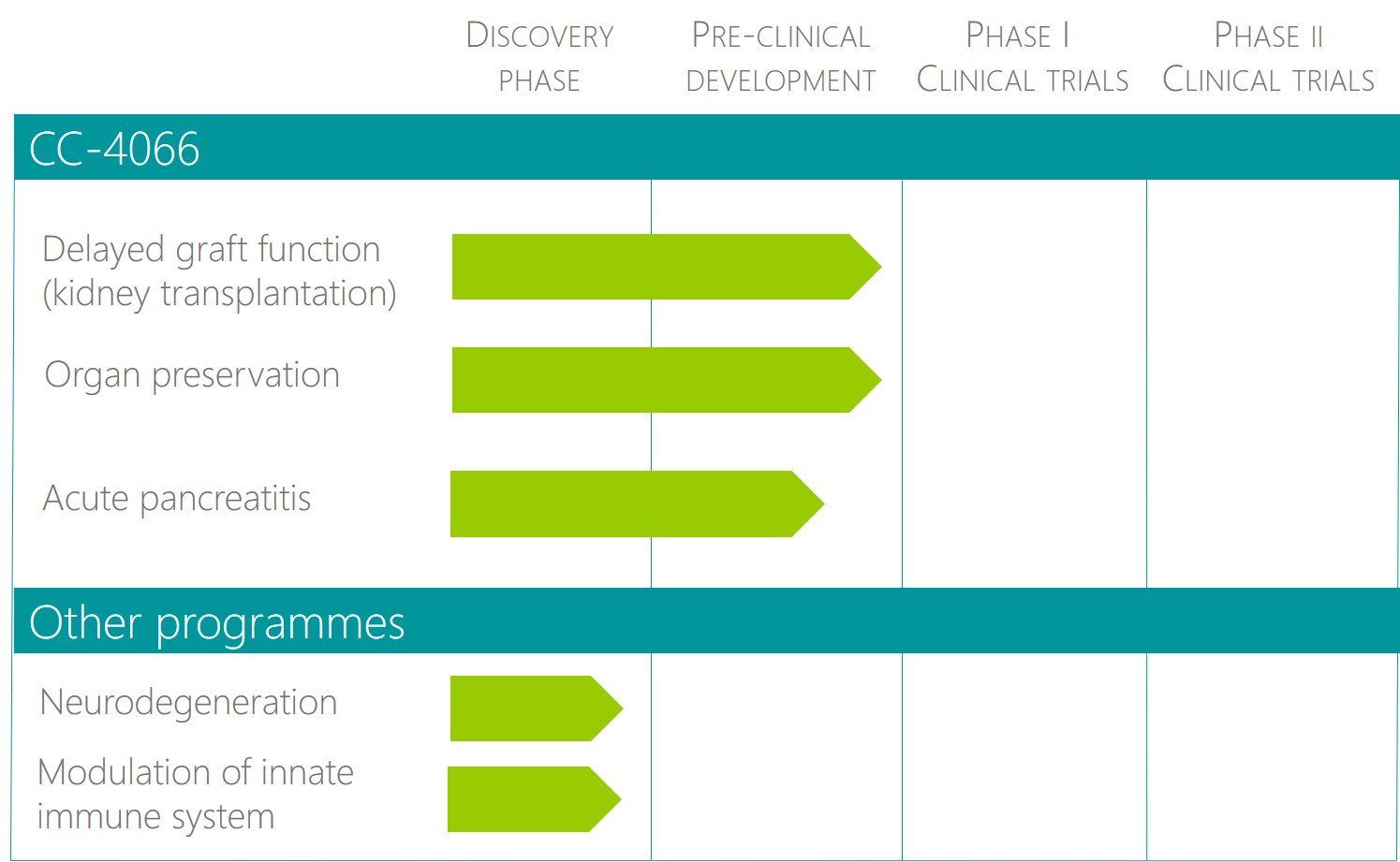
Pipeline
The strategy of Cypralis is focused on diseases representing an unmet clinical need where the treatment is of short duration and efficacy can be established on the basis of clear and accepted clinical endpoints in a relatively short timeframe. Acute kidney injury (AKI) represents such a disease. Administration of CC-4066 prior to an insult causing AKI has been shown in several test systems to provide excellent protection and we have chosen kidney transplantation as the first indication to provide proof of concept in humans. Once the protective activity is established there is a wide spectrum of diseases that can be treated by this drug.